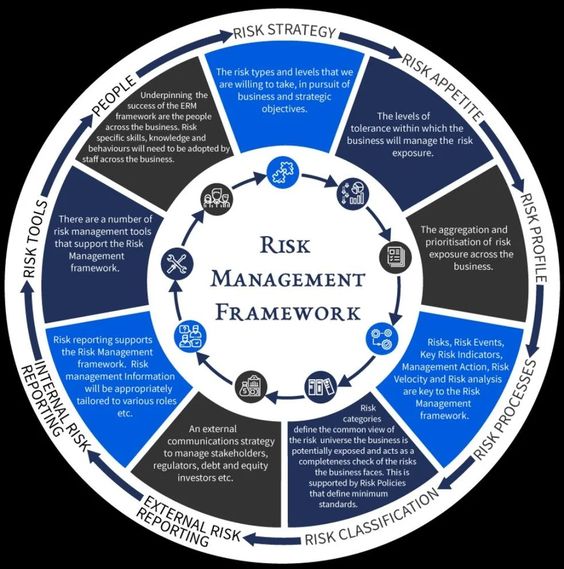
Understanding Quality Risk:
Quality risk is the probability of a product or service not meeting the expected quality standards. It is an assessment of the potential impact of a product or service not meeting the expected quality standards. Quality risk management is a systematic process used to identify, assess, and mitigate risks associated with the quality of products or services. Quality risk management is a critical component of any quality system.
Identifying Quality Risks:
The first step in quality risk management is to identify potential risks. This can be done through a variety of methods, such as brainstorming sessions, interviews with stakeholders, and reviews of existing processes. Once potential risks have been identified, they should be analyzed to determine their potential impact on the product or service.
Assessing Quality Risks:
Once potential risks have been identified, they should be assessed to determine the likelihood of them occurring and the potential impact on the product or service. This assessment should include an analysis of the potential risk and the potential consequences of the risk.
Mitigating Quality Risks:
Once the risks have been identified and assessed, they should be mitigated. This can be done through a variety of methods, such as implementing process changes, introducing new controls, or providing additional training.
Monitoring Quality Risks:
Once the risks have been mitigated, they should be monitored to ensure that they remain under control. This can be done through regular reviews and audits of the processes and controls in place.
Communicating Quality Risks:
Quality risks should be communicated to all stakeholders involved in the process. This will ensure that everyone is aware of the potential risks and can take appropriate action to mitigate them.
Reviewing Quality Risks:
Quality risks should be reviewed on a regular basis to ensure that they remain under control. This review should include an assessment of the potential risks and the potential consequences of the risk.
You might find these FREE courses useful
- Implementing a Risk Management Framework
- Program Risk Management in ClickUp
- Introduction to Risk Management
- A General Approach to Risk Management
- Risk Management Specialization
Documenting Quality Risks:
Quality risks should be documented to ensure that they are properly managed. This documentation should include the risk assessment, the mitigation plan, and any changes that have been made to the process or controls.